the Technology Interface / Fall 1997
A PRIMER ON HAND SOLDERING ELECTRICAL CONNECTIONS
by
David L. Pippen
dpippen@nmsu.edu
Assistant College Professor
New Mexico State University
ABSTRACT
Hand soldering techniques and various alloys used in the soldering process are discussed.
Some guidelines for selecting soldering alloys, characteristics of various solder types,
solderable metals, solder selection, the process of producing a good solder joint, and
desoldering are discussed. A summary of causes for poor solder connections is given.
WHAT IS SOLDERING?
Soldering is a process by which you can join metal items together by applying heat along
with special metallic alloys (solder) and allowing them to cool. This results in a metal bond
between the metals that is strong and has good electrical conductivity under harsh mechanical
environments.
WHAT IS SOLDER?
Solder is a metal alloy consisting of distinct percentages of two or more metals. In
electrical work, the alloy is usually tin (Sn) and lead (Pb). However, Silver (Ag), Zinc (Zn), and
Antimony (Sb) are used for special soldering alloys. Solder that uses lead has a lower melting
point than pure lead. However, some solders contain no lead.
- Each alloy composition has unique characteristics, eg., melting point, hardness,
and solid-to-liquid transition properties, and may or may not be eutectic.
- A eutectic alloy is a composition of one or more metals that has one sharp melting
point and no intermediate "plastic" stage. Non-eutectic compositions have a semi-liquid temperature range where the metal can be "worked" due to its plastic nature.
For example lead has a melting point of 621 OF (327 OC) and tin melts at 450 OF (232
OC). Alloying 63% tin with 37% lead forms a eutectic that melts at 361 OF (183 OC).
Note that this particular alloy's melting point is lower than either of the parent metals.
WHAT IS THE IMPORTANCE OF ALLOY TYPE?
- Every soldering application requires solder with particular characteristics.
Important characteristics include melting temperature, hardness, strength, and ease of
bonding the metals being soldered.
- Alloys are designed to fulfill particular soldering needs . Examples
- Automotive uses such as radiator, body and fender repair. SN30 (30% tin 70%
lead) or SN40 (40% tin 70% lead) are generally used. These solders have a wide
plastic range which allows the solder to be worked before it solidifies or melts. For
example, SN30 becomes completely liquid at 496 OF (258 OC). As temperature is
lowered below this point, it becomes plastic and workable until it solidifies at 361 OF
(183 OC).
- Plumbing - plumbers generally use solid SN50 solder in conjunction with an
externally applied flux. This 50% tin 50% lead alloy has a melting point of 414 OF
(212 OC) and becomes plastic at 361 OF (183OC). Some city ordinances now
prohibit the use of solders that contain lead for use in potable water systems so a
non-lead solder is used.
- Electrical Connections - The most popular solder alloys used for Electrical
connections are SN60 and SN63. The SN63 is eutectic and melts at 361 OF (183
OC); it has no plastic region. SN 60 is more general purpose, is less expensive,
and has a very small plastic region (about 8 O F). It also has a low melting point of
361 OF. These alloys allow rapid solder time that can prevent excessive
temperature being applied to the component and helps prevent "cold" and "fracture"
joints as discussed later.
- Common Soldering Alloys - Table 1 provides the melting temperatures of several
soldering alloy combinations.
TABLE 1 -
Melting Temperatures of Soldering Alloys
| Sn = Tin Ag = Silver
Pb = Lead Sb = Antimony |
Temperature at
which solder
becomes plastic |
temperature at
which solder
becomes liquid |
| %Sn
|
%Pb
|
%Ag
|
%Sb
|
OC
|
OF
|
OC
|
OC
|
| 0 |
100 |
. |
. |
. |
. |
327 |
. |
| 10 |
90 |
. |
. |
224 |
435 |
302 |
576 |
| 38 |
62 |
. |
. |
.183 |
361 |
242 |
468 |
| 48 |
52 |
. |
. |
183 |
361 |
218 |
424 |
| 60 |
40 |
. |
. |
183 |
361 |
188 |
370 |
| 63 |
37 |
. |
. |
Eutectic |
. |
183 |
361 |
| 100 |
0 |
. |
. |
. |
. |
232 |
450 |
| 95 |
. |
. |
5 |
232 |
450 |
238 |
460 |
| 35 |
63 |
. |
2 |
187 |
369 |
237 |
459 |
| 27 |
70 |
3 |
. |
179 |
354 |
312 |
594 |
| 40 |
57 |
3 |
. |
179 |
354 |
312 |
594 |
| 62.5 |
36.1 |
1.5 |
. |
Eutectic |
. |
179 |
354 |
| 96 |
. |
4 |
. |
Eutectic |
. |
221 |
430 |
| . |
97.5 |
2.5 |
. |
Eutectic |
. |
305 |
581 |
| .075 |
97.5 |
1.75 |
. |
Eutectic |
. |
310 |
590 |
WHAT IS THE PURPOSE OF FLUX?
Flux is a chemical cleaner which removes oxidation from metal surfaces so that a good solder-to-metal bond can be made.
- Flux must be applied to the surfaces at the bond point before soldering can be
successfully accomplished because copper and other materials rapidly oxidize upon
exposure to air , moisture, and heat. This oxidation is caused by exposure to the
oxygen in air/moisture and forms a non-conductive, non-solderable surface on the
metal. On copper surfaces, oxidation causes the bright shiny copper color of the non-oxidized surface to appear a dull, orange color.
- Flux comes in many grades. However, there are two general types: non-corrosive
and non-conductive (milder rosin types); and corrosive and conductive (very strong
acid types).
IMPORTANT: NEVER USE CORROSIVE AND/OR CONDUCTIVE FLUXES TO SOLDER
ELECTRONIC COMPONENTS.
- Solder can be manufactured with or without a core of flux.
o "Solid" solder has no core of flux. It is available in wire, bar, and other forms. An
external flux (usually liquid or paste) must be applied when soldering with these
non-flux solders. The flux is applied to the joint before the joint is heated. Many of
the fluxes are heat activated, meaning they do not remove the oxide layer except
while hot. Thus, the flux residue does not continue to act once it cools and it is
often not removed once the soldering has taken place. However, some fluxes
become tacky once they cool and thus can capture dirt and contamination. In time,
the normally high resistance cold flux that may exist between solder points can
develop into low resistance bridges causing circuit malfuntion. This type flux should
be removed once the soldering is complete. Commercial flux removers are
available. Isopropyl alcohol is often used.
o "Flux core" solder is usually in wire form. The center of the wire is filled during
manufacturing with the proper amount of flux required for soldering. Generally, no
additional flux is required when soldering with the flux core solders. The surfaces to
be soldered should not be visibly oxidized when soldering with these fluxes. Fine
grade steel wool or sandpaper are sometimes used to "polish" the surfaces prior to
soldering.
WHAT METALS CAN BE SOLDERED?
- Copper is readily soldered - Also, through chemical action, copper dissolves in the
solder to form an excellent bond.
- Beryllium copper, brass, and bronze are solderable, but not as easy as copper.
- Kovar and mild steel are solderable but with some difficulty.
- Chromium and stainless steel are extremely difficult to solder.
- Aluminum can be soldered using special fluxes and cleaning procedures.
Aluminum can have joints that look good (shiny and smooth), but actually be poor
structurally and have poor adherance.
WHAT IS THE SOLDERING PROCEDURE?
1. Use the right tools
- Wattage - 15 watts can be used for very small components and pads. 30 - 50
watts for larger components. Controlled heat irons are the best, but good
results can be obtained from the inexpensive fixed temperature irons that have
the proper tip wattage.
- Larger irons and "guns" should not be used except to solder very large
components. Do not use these high power instruments on electronic
assemblies or printed circuit boards.
- Soldering Iron Tip - The tip should be small enough so that the joint being
soldered can be easily seen, but large enough to quickly transfer the heat
required to raise the joint temperature to the solder melting point. The author
prefers a chisel (spade) tip that is between 0.05" and 0.08" across the spade for
general purpose soldering. Smaller tips are required for small pads and surface
mount components. The larger tip provides more heat which is required for
desoldering using desoldering braid or solder pump.
- Do not use acid core solder, corrosive fluxes, or conductive fluxes on
electronic equipment. Use mild fluxes such as contained in rosin core solder
or rosin flux.
- Use the correct alloy. SN63 is excellent for small, heat sensitive components
and printed circuit board pads. SN60 is an inexpensive excellent all-around
solder. Both are available with flux cores (usually rosin). Some fluxes are
sticky once activated by heat and thus should be cleaned off the board once
soldering is completed since they will accumulate dust/contaminants that may
cause an unwanted short or low resistance path at a later time.
- Use the correct diameter of solder especially for small component attach
points. (Author's opinions below)
- 0.020" dia. (25 gauge/.05 cm) rosin core or smaller - Very small. Excellent
for soldering very small printed circuit (PCB) board pads and hand
soldering surface mount components. Too small for a general purpose
bench solder. It can take excessive heating time to apply sufficient solder to
larger joints. The author has a small roll of 0.15 dia. solder on his bench but
uses it very infrequently.
- 0.031" dia.(21 gauge/.079 cm) rosin core - An excellent all around solder
for printed circuit boards and general kit building/electronic repair.
Inexperienced users may have some difficulty from forming solder bridges
between pads on junctions spaced 0.1" or less such as PCB integrated
circuit pads. This is the author's favorite size and is plentiful in his shop.
- 0.040" dia. (19 gauge/0.1 cm) rosin core - Good for larger connections
like tinning or connecting 14 gauge or larger wires to terminal strips,
connecting multiwires. Not good for PCB soldering because excessive
solder can be easily applied to pads increasing potential for undesired
solder bridges between points. The author has it available on his bench, but
hardly ever uses it.
- Larger diameter solder should be reserved for soldering large items like
very large stranded wires, soldering large items to aluminum chassis, etc.
The largest the author has is 0.047" dia. and he has never needed anything
larger for electronics projects. It is very seldom used.
- Side-cutters or "dikes" - Miniature side-cutters with spring to hold the jaws
open are ideal. The author prefers 5 - 51/2-inch for small wires and 6-inch or
greater for the larger wires. Most of the small cutters are for soft wire and can
be damaged cutting hard wires such as aluminum, iron or steel.
- Medium long-nose pliers - The same length as the side-cutters work fine. The
pliers should have round points so that component leads can be formed around
them when necessary. A pliar with 4" or longer nose is very useful for many
hard-to-reach applications.
- Desoldering equipment - A roll of desoldering braid and a vacuum desoldering
tool will cover most desoldering requirements. The desoldering pump is best for
removing large amounts of solder, and the braid for removing small amounts
and cleaning up solder holes in PCB's. The author often uses a pin vise with a
#65 drill bit to clean solder pad holes.
- Sponge - A damp sponge is very useful to keep the soldering tip free from
excess solder and contamination. Special solder tip cleaning pastes are also
available and do a good job of removing oxidized material from soldering iron
tips.
- Wire brush - A wire brush is useful to remove oxidation that may coat the
soldering tip after prolonged service. Be sure and tin the tip immediately after
brushing. Steel wool can also be used to clean the tip as well as
tarnished/contaminated component leads and surfaces to be soldered.
2. PREPARE THE SURFACES TO BE SOLDERED
- PCB's and surfaces. A thin film of oxide forms on bare copper that will be
detected as surface dullness and darkening of the copper. Thus, if bare copper is
to be soldered, it is very important to clean it with fine (0000) steel wool or
equivalent. The surface should be bright and shiny.
- Tinned surfaces do not normally need to be cleaned with steel wool, but all
contamination like dirt, oil, etc., must be removed. Rubbing alcohol or detergent
and water (if no components have been mounted) are good liquids to use.
- Use steel wool or fine sandpaper to clean leads of components stored for long
periods. Some components,
especially old resistors and
capacitors, will have tinned leads
that look dull rather than shiny. It is
a good idea to use steel wool to
clean them before soldering.
3. MOUNTING THE COMPONENTS ON A
PCB
- Mount components on non-foil
side with leads protruding
through the board to the copper
side.
- Place components against the board.
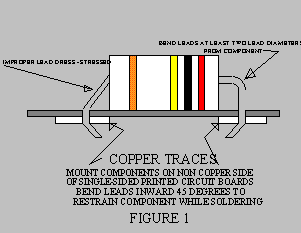
- Extend leads from component so that they are stress relieved (see Figure 1).
Bends should be at least two lead diameters from the component. Minimum inside
radius of the bend should be equal to a lead diameter. Part identification should be
visible with the part in place.
4. PREPARING THE SOLDERING IRON TIP BEFORE SOLDERING
- The solder tip must be applied to the joint at such an angle that the point of
contact can be observed during the soldering process. This is generally at
about a 45O angle.
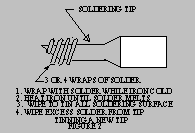
- The soldering tip must be clean and freshly
tinned before soldering.
It is extremely
important that a new, never been heated tip be
tinned immediately upon it reaching the
temperature that melts solder. Tinning is
accomplished by applying fresh solder and flux
to the tip and allowing all soldering surfaces to
become coated with solder. If tinning is not
done, then the tip will become oxidized and it
will be impossible to solder a good joint. This
oxidization is often difficult, if not impossible to
remove. Copper tips (copper color) can be filed, but do not file a tip that has been
plated (silver color). The tip should be
allowed to cool and then brushed vigorously
with a wire brush until the dark black/brown
oxidized material is removed. Sandpaper or
a fine file may be used to assist in this
process. Then reheat and tin as stated
above. Plated tips should not be filed or
sanded.
- If the tip has already been tinned and has
no oxidation, then clean the tip by wiping
on a damp sponge or other suitable
material before each connection is made.
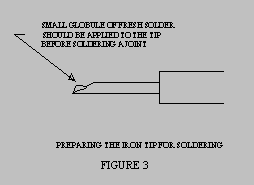
- Place a very small globule of fresh flux-core solder on the tip surface that will
be used as the point of contact with the parts to be soldered.
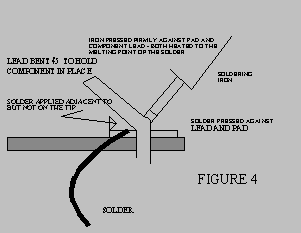
5. APPLY THE CORRECT AMOUNT OF HEAT AND SOLDER
- The soldering iron tip should be applied firmly to the metal part having the
greatest mass while also touching the part to be soldered to it.
- Apply heat until both the parts to be joined are sufficiently hot to melt the
solder.
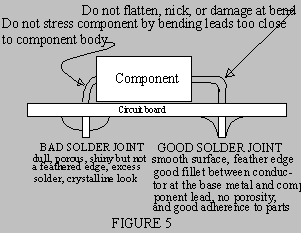
- Quickly apply solder adjacent to the tip, but not on the tip (Refer to Figure 5).
The solder should flow quickly around the components. Withdraw the tip as soon
as the joint is complete to avoid overheating the molten solder. The soldering
process should be completed within 2 seconds. If it takes up to 5 seconds then the
tip is too small, the iron too small, or the technique incorrect. The soldering tip
should be at a temperature of about 650O F (343 OC ).
- The surface temperature of both metals being soldered must be above the
solder melting point to expedite efficient wetting. Solder should not be permitted
to flow onto a surface cooler than the solder temperature; this will cause "cold"
joints.
Properly applied solder will
melt and flow smoothly around
the surfaces being soldered
producing a smooth, shiny
surface feathering out to a
smooth thin edge.
- A rounded, lumpy, dull,
irregular, or granular
appearance indicates improper
solder application.
6. DO NOT ALLOW JOINT TO MOVE
- Once the joint is soldered, it
is imperative that none of the
soldered parts be allowed to
move until the solder
solidifies.
- Premature movement will
cause the solder to fracture
at the component to solder
interface, thereby producing
a "fractured" joint. These
joints can be expected to fail
later while in service.
7. FINISHING THE SOLDERED JOINT
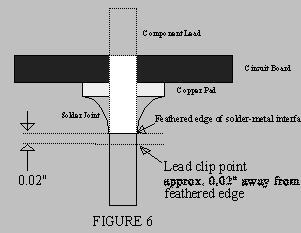
- Cut off excess leads (Refer to Figure 6). Do not clip into the feathered solder
edge. Leave a slight headroom so that the solder is not separated from the lead by
the shock forces imparted during cutting.
- Clean soldered board board
with flux remover or alcohol
after all joints have been soldered
and leads clipped. This may not
be necessary with some fluxes.
8. THINGS THAT MAKE A BAD
SOLDER JOINT
- Excessive solder applied to
joints pose the possibility of
unwanted solder bridges between adjacent joints.
- Applying too little solder allows low joint strength. Solder should completely fill
printed circuit board pads all around the component lead being soldered.
- Moving the joint before the solder solidifies will create a "fractured" joint. The
solder to metal bond is fractured just before solidification of the solder. This failure
may not be detected for years. This joint often looks non shiney and grainy,
especially at the interface.
- Applying too little heat will cause a "cold" solder joint. This joint may adhere for a
while, but can be expected to fail in time. This joint likely does not have feathered
edges where it interfaces with the pad and with the lead. It is generally has a solder
globule appearance rather than with feathered edges. It may be shiney.
- Applying too much heat to the tip will accelerate oxidation of the tip and cause the
solder to "roll" off it rather than wet it. A non wetted/tinned soldering tip will cause
excessive heat application time to circuit board pad and component. Pads can
even unadhere to the circuit board if too much heat is applied.
- Soldering a contaminated board or one with excessive oxide will produce a
non-functioning joint. The solder will tend to roll off the joint rather than bond.
- Soldering with no flux or too little flux will produce a non acceptable solder joint
Solder will often ball up and be globular in appearance.
- Soldering electronic parts with acid core solder, corrosive flux, or conductive
flux.
DESOLDERING
1. Many times, soldered components or wires must be removed.
2. There are at least two good ways to desolder components. One is by using a desoldering
pump and the other is by using a desoldering braid.
3. The desoldering tool is applied to a joint that has been heated to the solder's melting point.
A plunger is activated which "sucks" the solder into the tool's reservoir. If done properly, this
method can remove solder to the point that the component leads can be lifted away from the
metal to which they were joined.
4. If the joint does not have too much solder, then desoldering braid can be used to remove
the solder. This braid consists of copper braid impregnated with non-corrosive flux. The braid
is laid on top of the solder joint to be desoldered and the hot soldering tip applied to the braid.
Solder will flow towards the heat of the tip and away from the joint.
5. A good method of desoldering involves both of the above methods. Most of the solder can
be removed with the desoldering pump and any remaining with desoldering braid.
6. Desoldering irons are also available. These irons have vacuum devices attached to them.
The iron melts the solder and then the vacuum is energized which sucks the molten solder into
a reservoir for disposal.






